What problems can arise from using plant pathogen assays that were NOT specifically designed for cannabis plants?
Assays that were not specifically designed for cannabis matrices may cause false-negative or false-positive results
Anyone versed in cannabis testing can assure you, if you change the matrix, you must revalidate your technology. One cannot simply superimpose testing techniques used on other crops onto cannabis and assume the method’s function. One must revalidate the method on the new matrix.
In the case of plant pathogen qPCR assays, one must consider that the primers and probes may cross-react with the cannabis genome or other organisms native to the cannabis plant, creating false-positive or false-negative results.
 qPCR Basics
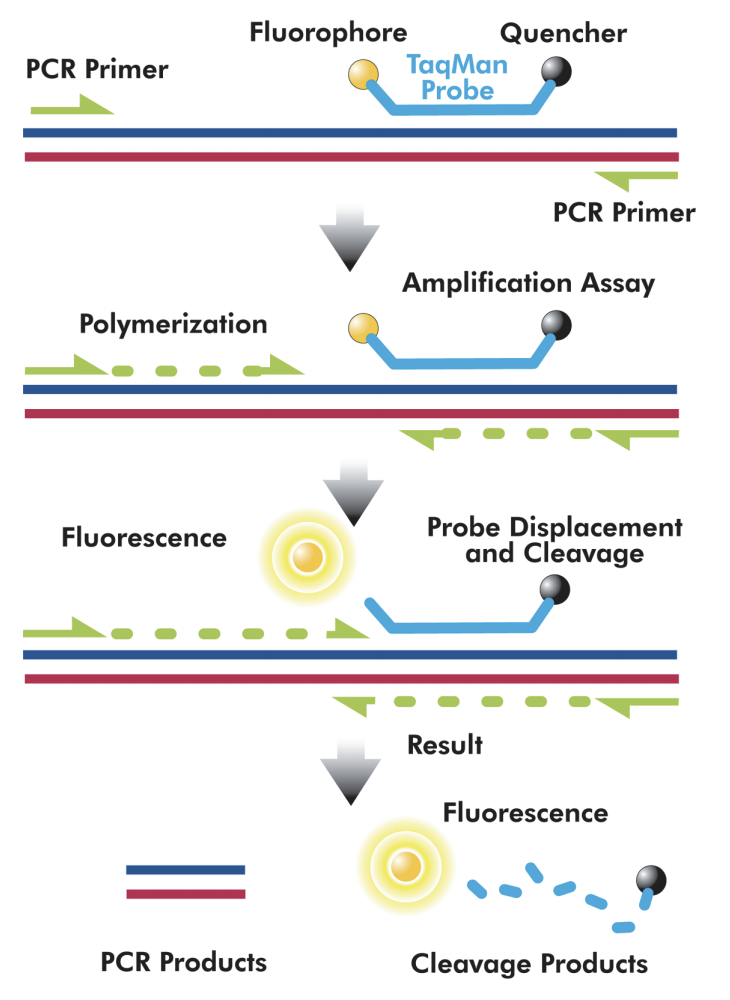
qPCR Basics
Kary Mullis invented PCR in 1988, for which he and his colleagues won the Nobel Prize for chemistry in 1993. PCR exponentially amplifies a segment of DNA to create exact copies in an abundance that allows for further analyses.
Each qPCR assay contains short DNA sequences called primers, which bind to either end of the target sequence and create a complementary strand. Probes also attach to the target sequence and when the PCR reaction cleaves the probe, it will emit a fluorescent signal that the instrument can detect.
Problems with non-cannabis specific primer and probe design
Assay manufacturers must design their primers and probes so that they will reliably attach to the target sequence AND they will not attach to non-target DNA that may be present in the sample.
Assays that were not specifically designed for cannabis matrices may use primers and/or probes that are similar to cannabis DNA or other organisms commonly found on cannabis. When that happens, it can produce the following problems:
- Primers and probes attach to non-target DNA. The primers will attach to and amplify non-target DNA, and the probes will emit a signal indicating a false-positive result.
- Primers attach to non-target DNA, but probes do not. PCR reagents are consumed by the amplification of the non-target DNA without producing a fluorescent signal. That can greatly reduce the sensitivity of the assay.
- Probes attach to non-target DNA, but primers do not. Probes are wasted attaching to non-target DNA and the PCR reaction will not cleave them off. That can greatly reduce the sensitivity of the assay.
Trust the experts at Medicinal Genomics
Medicinal Genomics was the first company to sequence the cannabis genome in 2011, and we developed our first cannabis-specific microbial safety assay in 2015. Our team understands the cannabis genome and the cannabis microbiome better than any other molecular assay developer on the market, and we have the publications to prove it. Our plant pathogen assays have been validated in the presence of cannabis in order to confirm there is no cross-reactivity with the cannabis genome.
