Enrichment is the only way to overcome sampling bias and ensure harmful pathogens can be detected in low levels.
Enrichment is the only way to overcome sampling bias. Cannabis producers collect a representative sample to submit to the lab for testing. The lab then breaks down that sample into smaller parts to use for the various types of analysis. Without enrichment, the subsample may not actually contain the harmful pathogen you need to detect. You can read more on sampling bias by following this link: https://www.medicinalgenomics.com/skipping-enrichment-puts-patients-lives-risk-lab-cutting-corners/
Enrichment is NOT to overcome poor sensitivity of qPCR assays. Another term for the sensitivity of and assay in the limit of detection (LOD). The LOD for our presence/absence assays are all detected to be ≤ 10 copies of a pathogens genome. Data that demonstrates the LOD of our assays begins on page 36 of our SenSATIVAx® and PathoSEEK® Manufacturer Validation document.
Which targets need enrichment?
Enrichment is required when regulations require < 1 CFU per gram of a target be present in a sample. Less than 1 CFU can be considered the same as testing for presence versus absence. Presence-absence targets require an enrichment step before subsampling and extracting DNA. If there are only a few pathogen cells present, whether fungal or bacterial, enriching and allowing them to grow and multiply is necessary to ensure they are captured in the final assay. The following figure depicts this very well, where the red dots represent cells of a pathogen that may be present in low levels.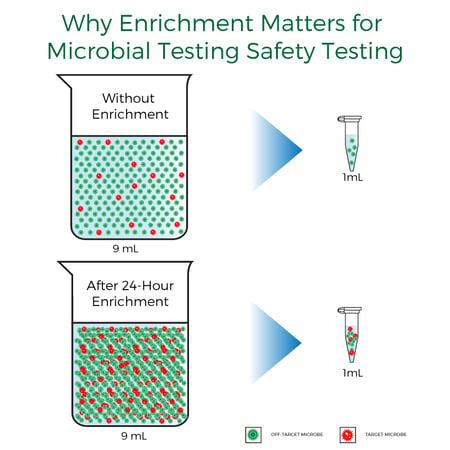
Can centrifugation be used as a substitute for enrichment?
No, the Medicinal Genomics team performed experiments to test whether centrifuging a sample prior to extraction is an adequate replacement for enrichment. The results show that centrifugation cannot be used in place of enrichment for identifying pathogens.https://www.medicinalgenomics.com/centrifugation-is-no-substitute-for-enrichment/What do regulators say about enrichment?
The State of Florida specifically requires enrichment. The following passage is taken from the FL MMJ Know the Facts Web Site:
Bureau of Cannabis Control (BCC) regulations call for an absence/presence test. Their document says a product batch passes if the product does not have any Shiga toxin–producing Escherichia coli, Salmonella spp., Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Aspergillus terreus at all in a 1-gram sample. https://bcc.ca.gov/law_regs/mcrsa_lab_ptor.pdf(2) For Microbiological Testing by quantitative polymerase chain reaction (qPCR), the Laboratory Batch must include one positive Quality Control sample able to detect 1 CFU per gram, one negative Quality Control sample, and one replicate Analytical Sample per Analytical Batch. Microbes with an Acceptable Limit less than 1 CFU per gram must undergo a 24-hour enrichment before testing.
https://knowthefactsmmj.com/2020/01/24/microbial-testing-methods/
What does The United States Pharmacopeia say about enrichment?
The USP requires enrichment when looking for the absence/presents of any specific organism: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q05a_pf_ira_34_6_2008.pdf
